Pharmacokinetic Studies of CSF and the Brain
For decades, the Salt lab has led the field in techniques for sampling fluids from the inner ear. Collecting small (0.5 – 1 µL) “clean perilymph” samples from the ear while minimizing contamination with blood, CSF or middle ear exudate is technically challenging [1] and requires the use of novel methods to isolate the collection point from adjacent tissues and fluids. Subsequent handling of the sample, to ensure accurate assay of the contents, also requires specialized procedures to ensure all the sample is transferred to the dilutant volume required for assay [2].
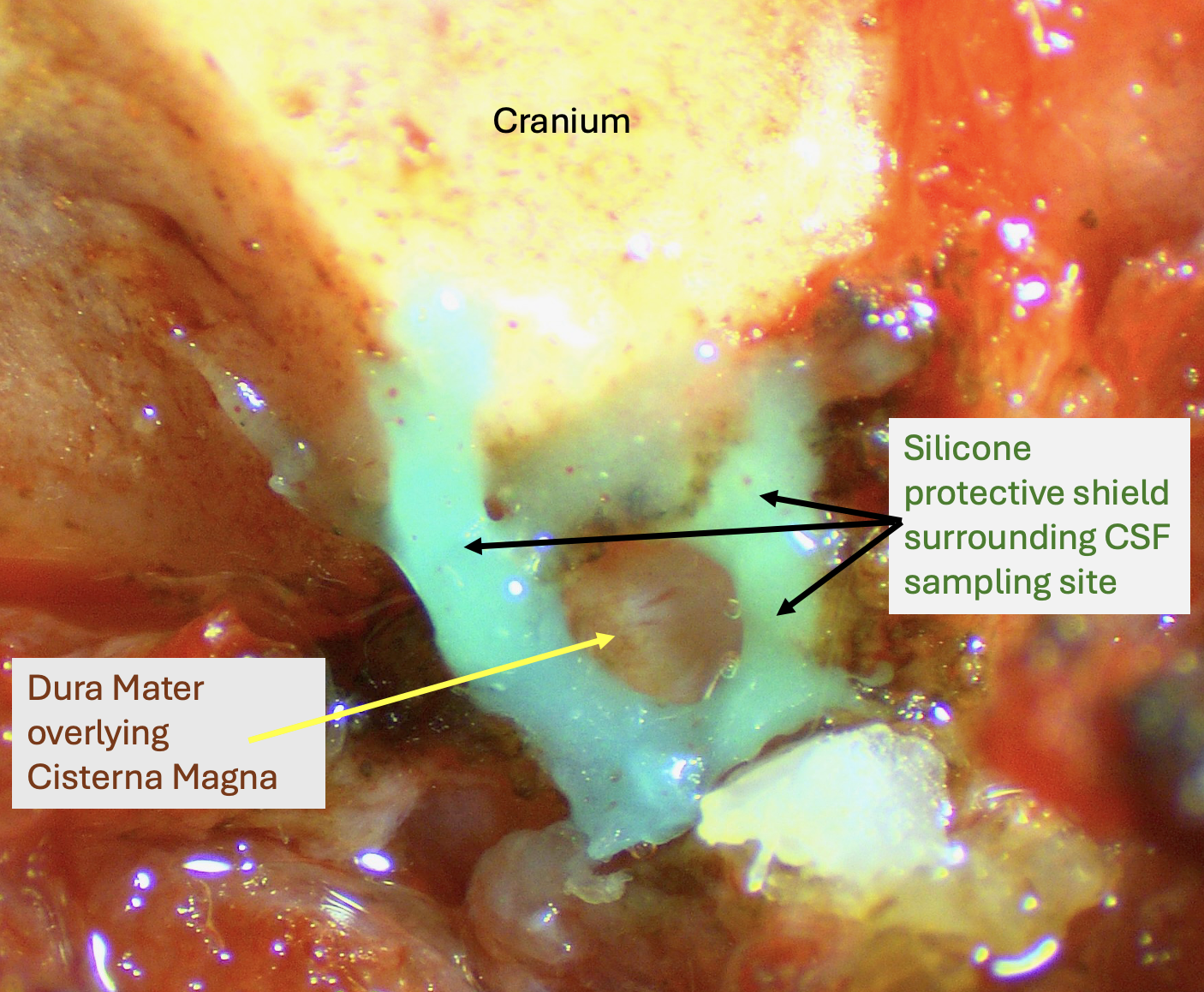
Some of our projects also required measurements of CSF concentrations of drugs or markers. From our perspective, although sampling CSF may initially seem simpler than sampling perilymph, there are some difficult problems to deal with. For CSF, the available fluid volumes are larger than for perilymph. Access to CSF, such as from the cisterna magna, is more straightforward. But collecting pure CSF without contamination with blood or plasma presents major challenges. This is especially important if the drug is being delivered systemically and plasma levels are much higher than CSF. We therefore adapted our “clean collection” techniques, to isolate the collection area from adjacent tissues and fluids, ensuring that the samples collected are of the highest purity possible. The figure below shows an example of the setup for CSF sampling in the rat. The dura mater is exposed caudal to the cranium and the surrounding tissues are coated with hydrophobic silicone cement. When the dura is perforated, clean CSF accumulates in the silicone "cup" which can be drawn into a sampling capillary. This minimizes contamination of the sample by contact with surrounding tissues and fluids.
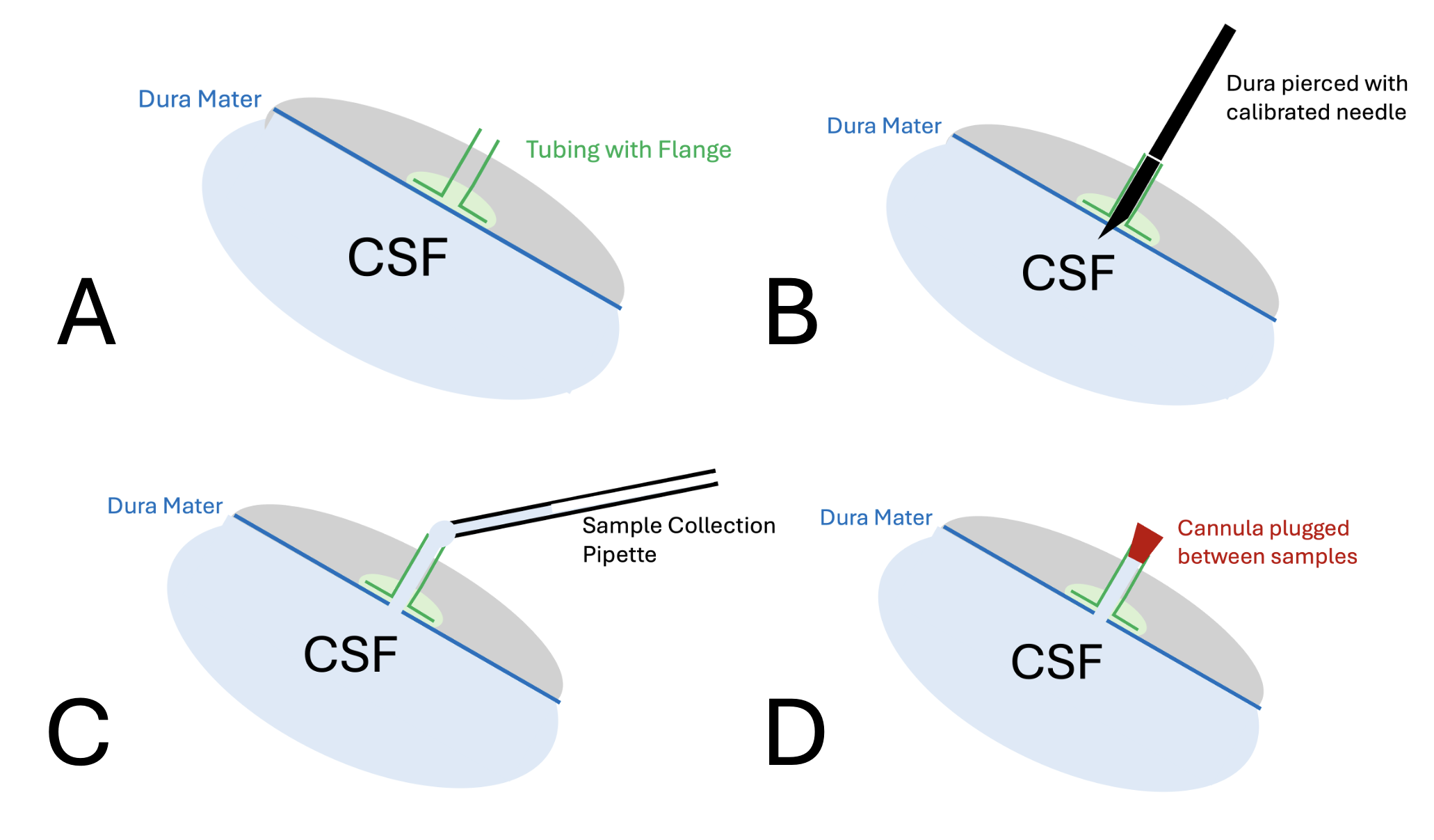
We also developed methods for the repeated collection of CSF samples at different time points in a single experiment. Since CSF leakage influences the kinetics of drugs, we allow CSF efflux to occur only for the brief period while collecting a sample and keep the CSF compartment closed during the periods between sampling. The steps of the technique are shown below. A piece of tubing with a flange is glued to the intact dura mater (A). The dura is pierced by passing a marked needle down the cannula so that the tip just perforates the dura (B). At this time, clean CSF can be collected by holding a collection capillary near the end of the cannula (C). A plug, held in a manipulator, is then used to close the cannula during the periods between taking samples (D). The volume of the plugged cannula is approximately 1 uL. If small (1 uL) samples are being collected, the first 1uL of each sampling can be discarded as not representing CSF within the cistern magna. All of the released and sampled CSF volume needs to be accounted for in the analysis of the results.
The availability of these clean collection techniques allows Turner Scientific to offer in vivo clean CSF sampling and analysis for studies of the CNS. CSF collection can be performed from a wide variety of species, including mice, rats, guinea pigs, and miniature pigs. We can also perform accompanying brain tissue analysis as required.
References
1) https://alecsalt.com/index.php/inner-ear-techniques/perilymph-sampling-from-the-cochlear-apex.
2) https://alecsalt.com/index.php/inner-ear-techniques/sample-handling-for-analysis
has experts fully trained in all aspects of CSF drug delivery and sampling.
Call (217) 602-0306 for assistance.