Important changes are happening in our field:
The FDA and NIH have recently revised their guidance related to animal studies used in drug development for human therapies.
FDA Announces Plan to Phase Out Animal Testing Requirement for Monoclonal Antibodies and Other Drugs.
Advanced Computer Simulations: The roadmap encourages developers to leverage computer modeling and artificial intelligence to predict a drug’s behavior. For example, software models could simulate how a monoclonal antibody distributes through the human body and reliably predict side effects based on this distribution as well as the drug’s molecular composition. We believe this will drastically reduce the need for animal trials.

The Entire News Release, April 10th, 2025: https://www.fda.gov/news-events/press-announcements/fda-announces-plan-phase-out-animal-testing-requirement-monoclonal-antibodies-and-other-drugs
NIH to Prioritize Human-Based Research Technologies
NIH is adopting a new initiative to expand innovative, human-based science while reducing animal use in research. Developing and using cutting-edge alternative non-animal research models aligns with the U.S. Food and Drug Administration’s (FDA) recent initiative to reduce testing in animals.
These technologies include Computational models which simulate complex biological human systems, disease pathways and drug interactions.

Full Article, NIH Record July 4th 2025: https://nihrecord.nih.gov/2025/07/04/nih-prioritize-human-based-research-technologies
In both cases, greater emphasis is being placed on computer models to simulate how specific drugs behave and how they distribute. For other body tissues and organs there are well-established drug simulation packages available (Simulations Plus; Simul8; Certara; Cadence, etc.) but none of these commercial packages deals with the complexity of the inner ear.
FluidSim provides the most sophisticated simulation available for drug distribution in the inner ears of experimental animals and humans. The algorithms used in FluidSim are based on over 40 publications in which drug or marker dispersal in the ear was compared for simulations and measured data (Salt & Turner, 2024; Supplementary Table S1). These studies allowed FluidSim to be trained and optimized for a wide range of delivery protocols, including systemic, intratympanic and direct injections into perilymph. The FluidSim program was written and developed by Alec Salt, who has been part of the Turner Scientific team since 2021. Simulations of drug delivery to the ear are not currently available from any other CRO world-wide. FluidSim leads the field in terms of quantifying drug distribution in the cochlea and vestibular systems of the inner ear.
Kinetic parameters for FluidSim are calculated from the molecular properties (WlogP and TPSA) of the specific drug, allowing a preliminary assessment of distribution characteristics even without measuring PK properties. The calculations allow PK experiments in animals to be well-designed and allow the measured data to be interpreted quantitatively to the maximum extent possible.
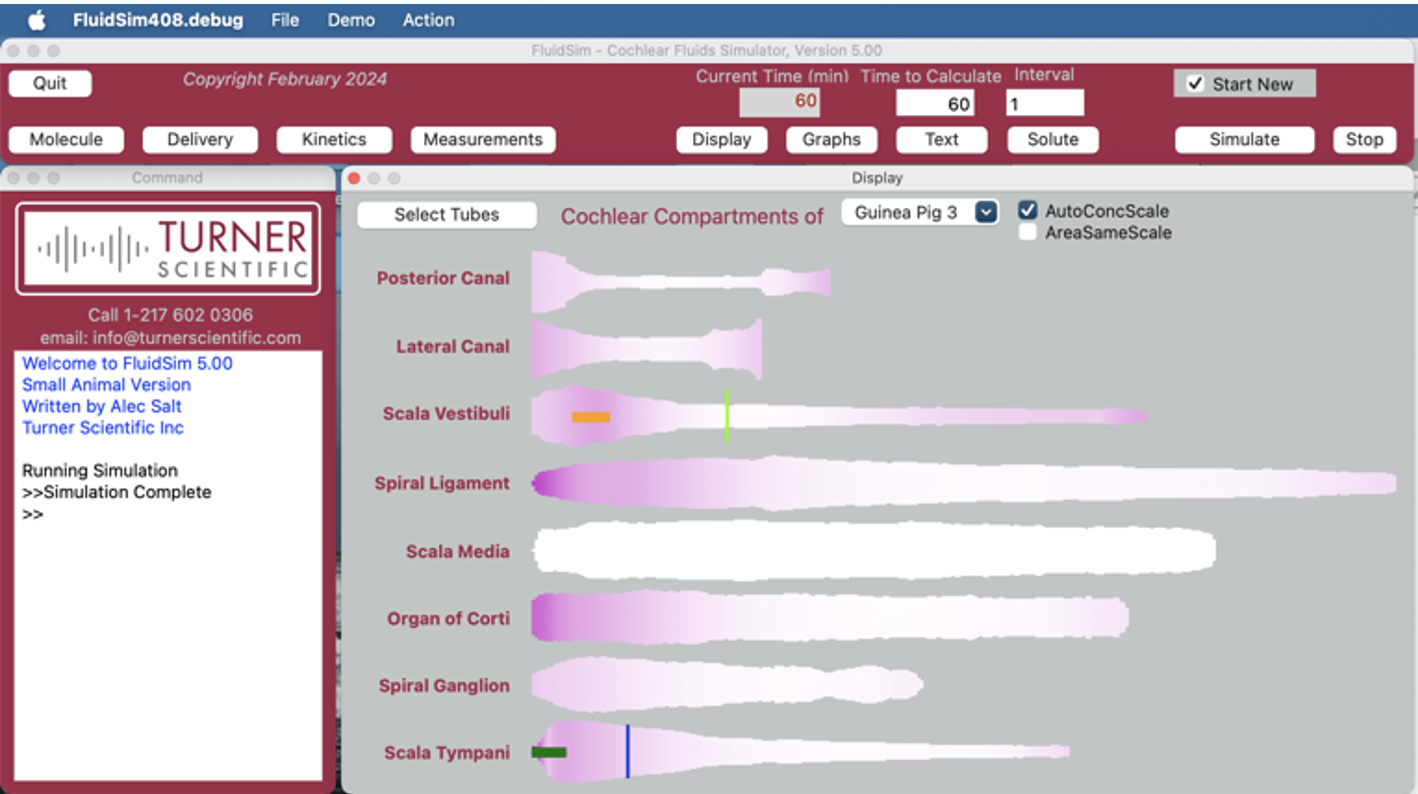
Figure 1: Screenshot of the FluidSim simulation program
Simulations with computer models representing the human biological system can now be used to:
1) Justify the need for specific animal studies
2) To predict drug amounts and distribution in the human cochlea based on existing knowledge or animal measurements.
3) To interpret PK measurements from animals and extrapolate to the human situation.
4) To compare delivery protocols for humans to optimize drug delivery.
Turner Scientific can assist you in proposal development using FluidSim simulation software.
The experts at Turner Scientific can advise on the best use of simulations to advance the development of therapeutics for therapy of the human ear. This can be a cost-effective approach that reduces animal usage through the three R's, which stand for Replacement, Reduction and Refinement.
Replacement: In some cases, simulation of a pharmacokinetic study is sufficient to show that use of a specific drug in a specific application will either be successful or unsuccessful, making the use of animal experiments unnecessary.
Reduction: Simulations of the planned pharmacokinetic study allow the experiment to be better designed, allowing data collection time points and drug dosing amounts to be optimized, avoiding unnecessary experiments.
Refinement: Analysis of measured data with FluidSim allows the maximum value to be extracted from the collected data and the experimental design to be refined over time.
If you need help incorporating inner ear simulations into your project or grant proposal, then set up a discussion call with us. Call +1 217 602-0306 or email
Recent Publications utilizing FluidSim:
Moatti A, Connard S, De Britto N, Dunn WA, Rastogi S, Rai M, Schnabel LV, Ligler FS, Hutson KA, Fitzpatrick DC, Salt A, Zdanski CJ, Greenbaum A. Surgical procedure of intratympanic injection and inner ear pharmacokinetics simulation in domestic pigs. Front Pharmacol. 2024 Jan 26;15:1348172. doi: 10.3389/fphar.2024.1348172.
Salt AN and Turner JG (2024) Drug selection for inner ear therapy. Front. Pharmacol. 15:1452927. doi: 10.3389/fphar.2024.1452927
Jung BT, Kuthubutheen J, Sharon JD, Foster AC, Erickson S, Peris H, De Juan E, Limb CJ, Farinas KC, Turner J, Henton A, Salt A. Plasma Concentration as a Proxy for Perilymph Drug Levels: Preclinical and Clinical Dexamethasone Measures with a Long-Acting Formulation for Precise Delivery to the Round Window Membrane. Otol Neurotol. 2025 Jan 1;46(1):80-87. doi: 10.1097/MAO.0000000000004336. Epub 2024 Nov 18. PMID: 39589784.
Salt AN, Turner JG. Dexamethasone Dosing of Human Perilymph Compared for Common Delivery Protocols using Inner Ear Simulations Audiology & Neurotology (submitted July 12, 2025): AUD-2025-7-