Providing guidance and supporting data for preclinical inner ear pharmaceutical therapeutics development.
Click here to go to the Turner Scientific Website
Here are the main 10 steps in the preclinical pipeline:-
1) Consideration of the physical properties of the therapeutic molecule(s)
Analysis of the physical properties of the molecule allows pharmacokinetic predictions and formulation considerations before any experimental studies start. Consideration of suitability of the molecule for oral, intravenous, intratympanic or intracochlear dosing pathways. Computer simulations (with FluidSim software) of drug entry and distribution characteristics likely in animal models (mice, guinea pigs, non-human primates and others) and of potential distribution characteristics in the human. Initial determination of whether it will be possible to generate therapeutic concentrations of the drug at the site(s) where it is needed in the human inner ear.
In-House Techniques:
Molecular properties calculation (WLOGP, TPSA, etc)
FluidSim Cochlear Fluids Simulator
2) In vitro assay of molecule efficacy in mouse organ of Corti explants
Approximates the in vivo condition, with accurate delivery of therapeutic molecule.
Cost-effective initial demonstration of drug efficacy and/or lack of toxicity.
Initial dose-response assessment of the drug.
Protection assays – protection against hair cell damage induced by chemical toxins such as gentamicin, cis-platin or cyclodextrin.
Drug toxicity assessment, establishing the upper level for therapeutic concentration.
In-House Techniques:
Neonatal mouse organ of Corti explants
Confocal microscopy
Quantification of hair cells, ribbon synapses, spiral ganglion cells, neurites
Specialty staining and imaging
3) In vivo efficacy in the mouse ear
Initial, low-cost assessment of how the ear responds to the drug. Protective influence against noise damage or chemical toxins can be assessed according to the mode of drug action. Local toxic influences (if any) to middle ear structures and to the inner ear can be evaluated
In-House Techniques:
ABR (auditory brainstem responses) - measure of synchronized electrical responses to trains of tone pips/clicks. Accepted by the FDA and scientific field as a standard “hearing” test in both preclinical and clinical research.
DPOAE (distortion product otoacoustic emissions) – measure of outer hair cell responsiveness. Often used in both preclinical and clinical research.
Automated, reflex-based behavioral audiometry – hearing loss, hyperacusis, tinnitus, presbycusis.
Drug delivery orally, systemically (IP, IV) or locally by intratympanic or by direct perilymph injection (into the posterior semi-circular canal).
Endocochlear potential measurement.
In-vivo evoked potential surface electrode measurements
In-vivo single and multi-cell recording techniques
Confocal microscopy
Otic histopathology (frozen or paraffin-embedded H&E)
Quantitative immunochemistry
Off-Site Techniques:
Mobile ABR/DPOAE systems
4) In vivo drug pharmacokinetics in the mouse ear
Determination of drug concentration being generated in the perilymph or in cochlear tissues by the chosen delivery technique. Measurements allow formulation development to generate optimum drug levels. The time course of drug levels in the inner ear can be established and dosing regimens compared. Computer simulations of the experimental findings can be performed to quantify kinetic variables (important in formulation development) and to refine predictions for the dosing strategy in larger animals or humans. Although PK studies can be combined with efficacy studies (#3 above) there are usually constraints in which PK studies require sampling at shorter time points, when drug is still present, while efficacy studies may require longer time points. Whether the two types of measurements can be combined depends on the specific measurements required.
In-House Techniques:
Drug delivery orally, systemically (IP, IV) or locally by intratympanic or by direct perilymph injection (into the posterior semi-circular canal our through round window membrane).
Perilymph sampling from the posterior semicircular canal. Yields approximately 1 uL sample per ear, representing the entire perilymph contents of the mouse ear.
Confocal microscopy
Otic histopathology (frozen or paraffin-embedded H&E)
FluidSim simulations for the mouse ear.
5) Efficacy in the guinea pig ear
A common next step is to evaluate the drug performance in a larger ear with hearing sensitivity more comparable to the human. The guinea pig is common choice, allowing more detailed hearing and pharmacokinetic measurements (#6 below) than are available in the mouse. Protective influences of the drug against noise damage or chemical toxins can be assessed according to the mode of drug action. Local toxic influences (if any) to middle ear structures and to the inner ear can be evaluated.
In-House Techniques:
ABR (auditory brainstem responses) - measure of synchronized electrical responses to trains of tone pips/clicks. Accepted by the FDA and scientific field as a standard “hearing” test.
DPOAE (distortion product otoacoustic emissions) – measure of outer hair cell responsiveness. Often used in both preclinical and clinical research.
Automated, reflex-based behavioral audiometry
Drug delivery orally, systemically (IP, IV) or locally by intratympanic or by direct perilymph injection (into the posterior semi-circular canal).
Endocochlear potential measurement.
In-vivo evoked potential surface electrode measurements
In-vivo single and multi-cell recording techniques
Confocal microscopy
Otic histopathology (frozen or paraffin-embedded H&E)
Quantitative immunochemistry
Off-Site Techniques:
Mobile ABR/DPOAE systems
6) In vivo kinetics in guinea pig ear
The inner ear of the guinea pig is substantially longer than the mouse which is of major importance to drug delivery as shown here. It is in the guinea pig where some of the difficulties in delivering drugs to the human cochlea become apparent. Simulations show that for longer cochleas, drug distribution from the base (where drug typically enters perilymph) to the apical regions is limited. It primarily depends on how rapidly the drug is lost to the vasculature as it diffuses along the spiral fluid spaces. A drug that leaks easily to the vasculature may never reach the more apical regions of the cochlea, where regions responding to sound frequencies important to speech discrimination are located. As the fluid spaces of the guinea pig cochlea are larger than the mouse we can perform “sequential sampling” of perilymph. This method collects many small (1 or 2 uL) samples, one after the other, either from the cochlear apex or from the lateral semi-circular canal. The samples are each analyzed separately, allowing drug gradients along the ear to be demonstrated and quantified. The guinea pig also allows other measures which are relevant under different circumstances, such as middle ear contents plasma and CSF concentrations over time. Defining middle ear drug concentration with time is commonly essential to interpreting perilymph PK kinetics quantitatively. Sequential sample data provide an excellent basis for simulations of drug distribution as a function of distance and time using FluidSim, such as those shown below.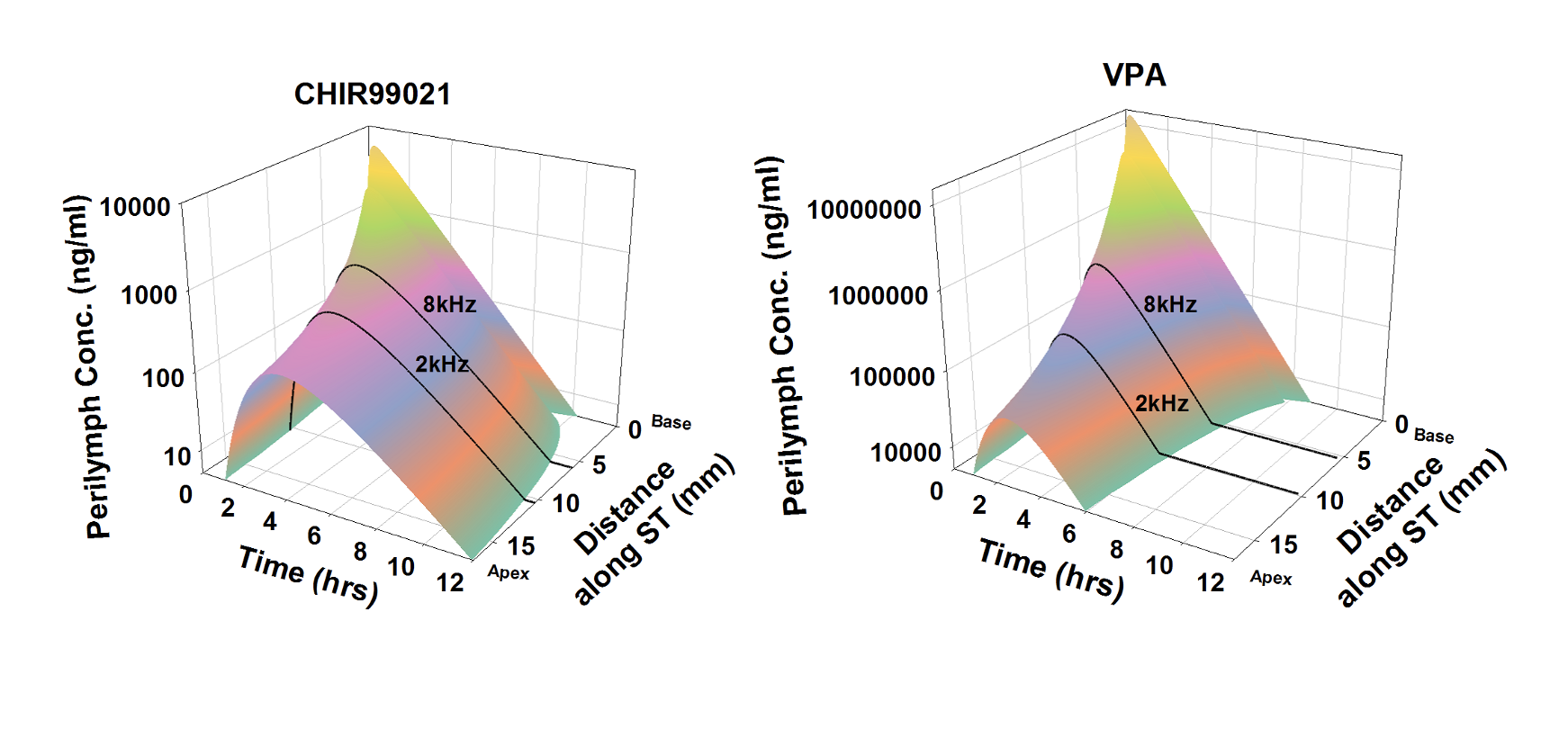
Calculated distributions of CHIR99021 and VPA as a function of distance and time in the guinea pig cochlea based on the measured distribution of drug 1 hour and 3 hours after round window (RW) niche applications. Calculations include kinetic processes of the middle ear, entry into perilymph and elimination from perilymph. From McLean et al., (2021) Supplementary Material.
In-House Techniques:
Drug delivery orally, systemically (IP, IV) or locally by intratympanic or by direct perilymph injection (into the posterior semi-circular canal).
Endocochlear potential measurement.
Perilymph sampling of single, large samples. 5uL from the cochlear apex represents the entire scala tympani contents. 15 uL from the lateral semi-circular canal represent entire perilymph of the ear.
Sequential sampling from the cochlear apex (described in detail here https://alecsalt.com/index.php/inner-ear-techniques/perilymph-sampling-from-the-cochlear-apex). Collecting 8 x 1 uL samples from the cochlear apex defines the drug gradient along scala tympani. Collecting 10 x 2 uL samples from the lateral semi-circular canal defines scala tympani-scala vestibuli (whole cochlea) gradients.
FluidSim simulations for the guinea pig ear.
In-vivo evoked potential surface electrode measurements
In-vivo single and multi-cell recording techniques
Confocal microscopy
Otic histopathology (frozen or paraffin-embedded H&E)
Quantitative immunochemistry
7) Drug distribution predictions for the human
The human cochlea is longer than that of the guinea pig (see here) which means that drug gradients along the ear will be greater for the human than for the guinea pig. Simulations with FluidSim take into account the anatomic dimensions of the inner ear for a number of species, including humans, so that drug distribution along the human cochlea can be predicted. As can be seen in the figure below, the distance the drug distributes from the base, where it enters, is highly dependent on molecular and pharmacokinetic properties. Such simulations give the best indication of where the therapeutic agent will reach in the cochlea.
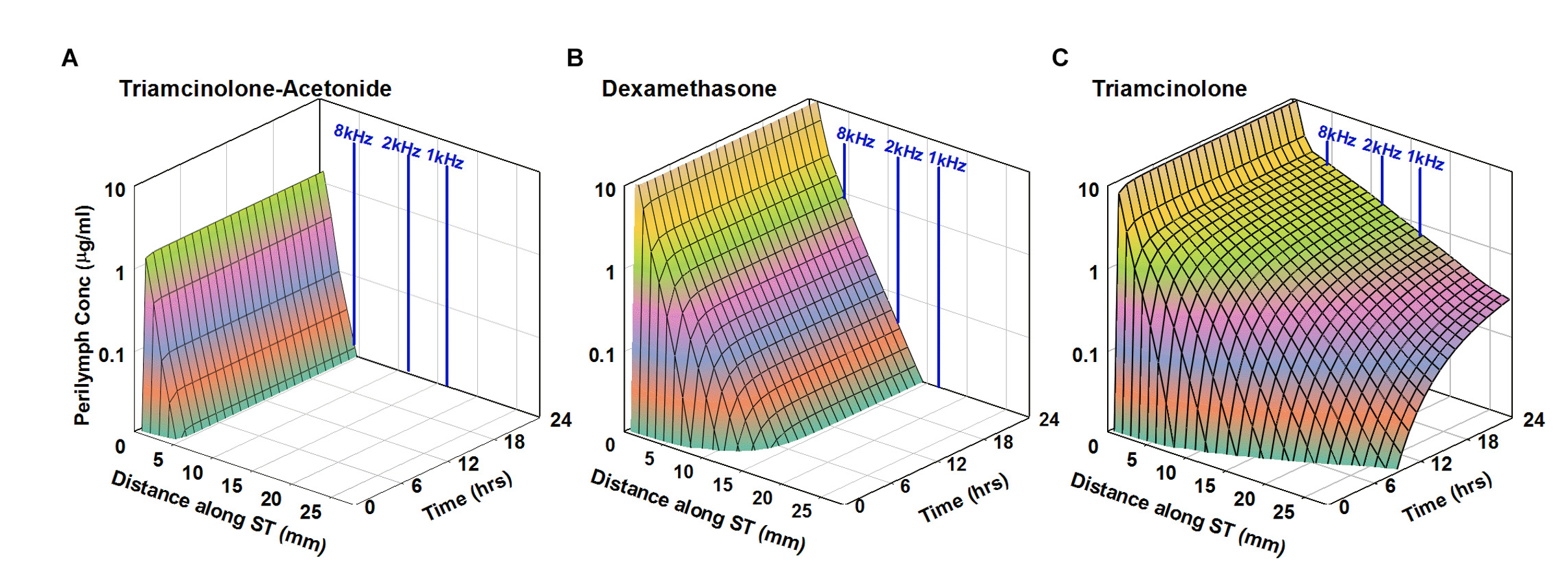
Simulation of intratympanic applications of triamcinolone-acetonide suspension (A), dexamethasone suspension (B), and triamcinolone suspension (C) to the human ear. Entry and elimination kinetics are based on perilymph measurements in guinea pigs. ST elimination half-times were 12, 44, and 700 min for triamcinolone-acetonide, dexamethasone, and triamcinolone, respectively. Triamcinolone-acetonide and dexamethasone only distribute apically to a limited degree before reaching a steady state, unlike triamcinolone which reaches the cochlear apex. From Salt et al., (2019).
In a limited number of cases, we have been able to compare model predictions for the human with measurements from samples of human perilymph. Panel A of the figure below shows a FluidSim prediction of the spatial and temporal distribution of dexamethasone in the human ear based on pharmacokinetic parameters established in guinea pigs (Salt et al., 2018). In panel B, the prediction is compared with measurements of perilymph samples from humans reported by Bird et al. (2011). While the human data exhibit high variability, the model prediction lies “near the middle of the pack” with only minor parameter adjustments required to provide a best-fit to the data.
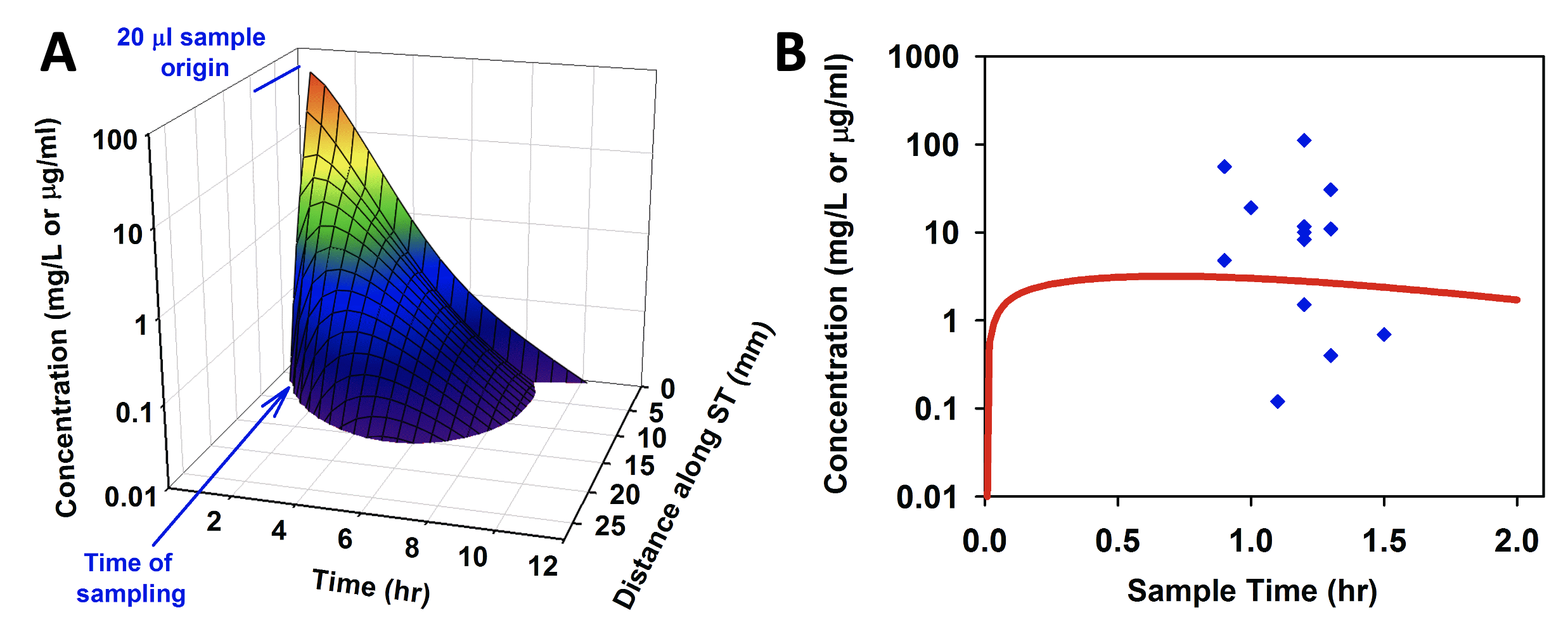
Comparison of model prediction (A, and red line in B) of dexamethasone distribution in the human, with measured perilymph values (B, blue points, Bird et al, 2011). Adapted from Salt et al., (2018).
In-House Techniques:
FluidSim simulations for the human ear.
Assistance with setting up and running your own simulations
Program customization for specific purposes (such as delivery approaches))
8) Efficacy and pharmacokinetics studies in non-human primates (NHP)
Non-human primate studies (commonly in Macaque monkeys) are regarded as the “closest-thing-possible” to human studies. NHP studies are performed off-site in collaborator’s facilities. Functional testing is performed with mobile ABR systems. We can also assist with study planning to minimize the number of animals required. The inner ear of the macaque is quite similar in dimensions to that of the guinea pig. Scala tympani length is 17.4 mm compared to 17.1 mm in guinea pigs; scala tympani volume is 7.78 uL compared to 5.97 uL in guinea pigs. However, it is much more difficult to perform PK studies as fluid sampling is much more difficult (Manrique-Huarte et al., 2021). This underscores the need to plan such studies extremely carefully, especially if pharmacokinetic measurements are involved.
In-House Techniques:
FluidSim simulations for the macaque inner ear.
Off-Site Techniques:
Mobile ABR/DPOAE systems
9) NIH Grant Applications
To advance the development of therapeutics, all sources of funding should be considered. Scientists in our group have extensive experience and solid track records obtaining NIH and other government and foundation funding and in reviewing Federal and other grant applications. We can review and advise on R01, SBIR (small business innovation research) and STTR (small business technology transfer) grant applications. We can act as consultants or as co-investigators, providing the site where data will be collected.
10) FDA submission guidance
Scientists in our group have been involved in FDA submissions and reviews, enough to offer a limited degree of guidance on:
FDA Pre-sub (pre-submission) for IND (investigational new drug) or NDA (new drug applications).
Physiologically based pharmacokinetic (PBPK) analyses submissions.
IVIVC (in vitro/in vivo correlations).
ANDAs (abbreviated new drug applications) and ANDA supplements.
PK Models and Simulations.
Click to go to the Turner Scientific Website
Reference Citations
Bird PA, Murray DP, Zhang M, Begg EJ. Intratympanic versus intravenous delivery of dexamethasone and dexamethasone sodium phosphate to cochlear perilymph. Otol Neurotol. 2011; 32(6): 933–6.
Manrique-Huarte R, Linera-Alperi MA, Parilli D, Rodriguez JA, Borro D, Dueck WF, Smyth D, Salt A, Manrique M. Inner ear drug delivery through a cochlear implant: Pharmacokinetics in a Macaque experimental model. Hear Res. 2021 404:108228.
McLean WJ, Herby J, Loose C, Hinton A, Lucchino D, Yang-Hood A, Schrader AD, Ohlemiller KK, Salt AN, Hartsock JJ, King S, Jackson L, Rosenbloom J, Aitee G, Bear M, Runge C, Gifford R, Lee D, Rauch S, Langer R, Karp J, LeBel C Improved Speech Intelligibility in Subjects With Stable Sensorineural Hearing Loss Following Intratympanic Dosing of FX-322 in a Phase 1b Study, Otol Neurotol. 2021 42(7):e849-e857
Salt A., Hartsock J, Piu F, Hou J. Dexamethasone and dexamethasone-phosphate entry into perilymph compared for middle ear applications in guinea pigs. Audiol Neurootol. 2018;23:245-257.
Salt AN, Hartsock JJ, Piu F, Hou J. Comparison of the pharmacokinetic properties of triamcinolone and dexamethasone for local therapy of the inner ear. Frontiers in Cellular Neuroscience 2019; 13, 347. https://www.frontiersin.org/article/10.3389/fncel.2019.00347